Make big Rochelle salt crystals from seeds
On this page I'll show you how to grow a large crystal from a smaller seed crystal. You can see how to grow a small Rochelle salt seed crystal here. This method should work for other types of crystals besides Rochelle salt, but I've used it only for Rochelle salt so I can't guarantee it.
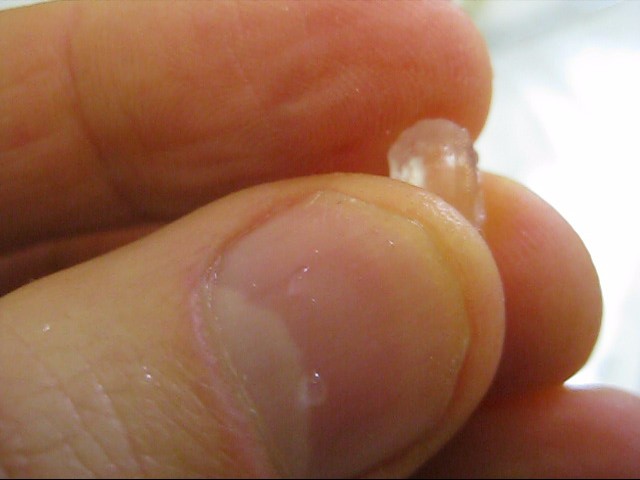

Note that I didn't record anything while making my bigger crystal from a seed crystal and instead referred people to someone else's webpage for instructions on how to make it. However, that webpage is now gone, so I've put together this webpage instead. Sadly that means there are no photos of the process.
Some basics - Saturation, supersaturation and controlling temperature
The basic idea is to create a supersaturated solution and grow the seed crystal in it. What's a supersaturated solution? Well, we'll be disolving some substance in distilled water. If you're growing a Rochelle salt crystal than the substance would be Rochelle salt. You may have done this when stirring salt, sugar or coffee in water. We'll keep adding that substance and stirring until no more can be disolved. Any additional substance we add just stays as the substance sitting in the bottom -- it doesn't disappear into the water. At that point we say the solution, the water and whatever we've disolved into it, is saturated. It's saturated when we can't disolved any more.
But, if we heat up the solution, then we can disolve some more into it. And when it cools down again, the solution is now supersaturated.
Why do we need a supersaturated solution? Why can't we just put the seed crystal in a saturated solution? A solution that's saturated has a stable mix of the distilled water and the substance. It's at equilibrium. Since it's a stable mix, there's no reason for the substance to come out of the solution and grow on the seed crystal.
But if the solution is supersaturated, some of the substance will come out of the solution and grow on the seed crystal. At some point so much of the substance may come out of solution and grow on the crystal that the solution is just staturated again, at equilibrium. No more will come out at that point and the crystal will stop growing.
There is a danger at that point if you allow the solution to heat up. If you heat up a solution that's saturated, then the substance will actually come from the crystal and disolve back into the water. Your crystal will shrink!
So heating and cooling are important factors here. You'll have to control the temperature carefully for this to work.
Making the supersaturated solution
Here are two methods for making a supersaturated solution.
When heating a solution you should use a double boiler. For example, I put a metal pot on the stove with water in it. In that I put a clean 500ml/2 cup pyrex measuring cup to act as the container. The solution goes in the container. A glass beaker can be used instead of the measuring cup.
Method 1
- Prepare double the amount of the substance (e.g.
Rochelle salt) that you normally would.
Example:
If you'd normally put 20 grams in 100ml of water, instead prepare 40 grams for putting in the 100 ml of water. - Put room temperature distilled or demineralized water into the container.
- Put in a little of the substance at a time in the water while stirring until it disolves. Keep doing this until you put some in and it no longer disolves.
- Gently warm the solution while putting a little of the substance at a time while stirring. Since the temperature is higher, more should disolve this time.
- Once all of the substance has been disolved into the solution, stop and remove from the heat.
- Let it cool to room temperature.
The result is a supersaturated solution.
Method 2
- Put an appropriate amount of distilled or demineralized water in the container in the double boiler.
- Heat it to around 15 to 20 degrees celsius (60 to 70 fahrenheit) above room temperature.
- Put some of the substance (e.g. Rochelle salt) in and stir it until it disolves.
- Keep adding more of the substance and stirring it until it no longer disolves.
- Increase the heat some more until stirring disolves the substance.
- Keep doing this until all of the substance has been disolved in the solution.
- Remove from the heat and let it cool to room temperature.
The result is a supersaturated solution.
Preparing the crystal growing environment
As pointed out above, controlling the temperature is very important for making the bigger crystal from the seed crystal. You'll need to find a room where the temperature stays very constant. I emptied out a shelf in a linen closet and used that as the room.
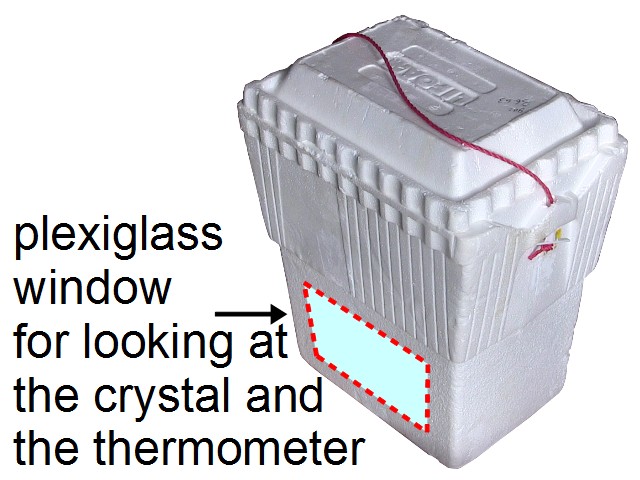
I then got a styrofoam container that would fit my glass container (see the diagram below). Mine was around 30cm/12 inches tall by 22cm/9 inches wide by 18cm/7 inches deep. I then cut a hole in the side that would allow me to see plenty of the glass container inside so I could watch the progress of the crystal growth without opening the container. I then cut out a piece of 1/8" or 1/4" thick plexiglass large enough to cover the hole and used silicone chaulking to attach it to the container. Some tape will probably do instead but test the tape first to make sure it will stick well. Again, I didn't take photos so you'll have to go by the diagram.
I then tied one end of a short length of fishing line to a stick (Examples: a popcycle stick, or two straws taped together, or a plastic knife). I tied the other end around the seed crystal. Mine was a Rochelle salt crystal made using the method outlined on my webpage about how to make Rochelle salt crystals. I tied it such that it would sit in the middle of the supersaturated solution in the container as shown in the diagram. I taped the stick in place.
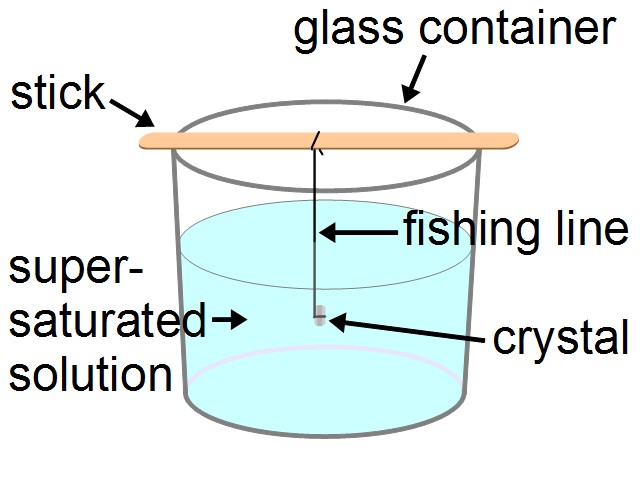
Then I covered the container with either cellophane or aluminum foil, I don't recall which. This is just to further prevent dust or drafts from flowing air from interfering or affecting the temperature.
That was then put in the styrofoam container in the closet. I also put a thermometer in the container in a position that allowed me to see it. The cover was put on the container and the cover taped firmly in place. The side with the plexiglass window was facing out of the closet so that to check on it I could just open the closet door and look in on it without disturbing anything. You may need a flashlight to see in well.
Growing the crystal
Check it as seldom as possible, maybe only a few times a day since you want to keep the temperature in the closet as constant as possible. Keep looking in on it until the crystal stops growing. Mine took around a week.
Gradually the crystal will grow by removing the substance from the solution. The solution will become less and less supersaturated. The crsytal will stop growing when the solution is at equilibrium, it is no longer supersaturated, but is just saturated. See the section above explaining these terms.
What if the crystal shrinks?
Your crystal may shrink, or disappear. This can happen if the temperature gets warmer. In that case the solution can become unsaturated. That will cause some of the substance to leave the crystal and go back into the solution. This is why you have to control the temperature.
Resupersaturating the solution
When the crystal stops growing, you can make it grow larger still by supersaturating the solution again.
First, remove the crystal. You want to keep it clean so don't just sit it on a table. Suspend it in air in another container, for example. You can also clean the crystal before putting it back. Make sure it has dried, but don't touch it with your fingers. Remove any bumps from the surface and any small crystals on the fishing line.
To resupersaturate the solution, you can do one of the following.
- Reheat it to reduce the amount of water. Then let it cool back to room temperature.
- Just let it sit uncovered and let the water evaporate without heating. This takes longer but results in a better crystal.
- Reheat it and disolve in more of the substance. Then let it cool back to room temperature.
Once you have a newly supersaturated solution, put the crystal back in and put it all back in the styrofoam container in the closet.
You can keep resupersaturating the solution each time the crystal stops growing as often as you want.
To get a crystal with good symmetry you can rotate it while it grows. This can be done 1 to 4 times a day.
