How to use a hydrometer to measure specific gravity
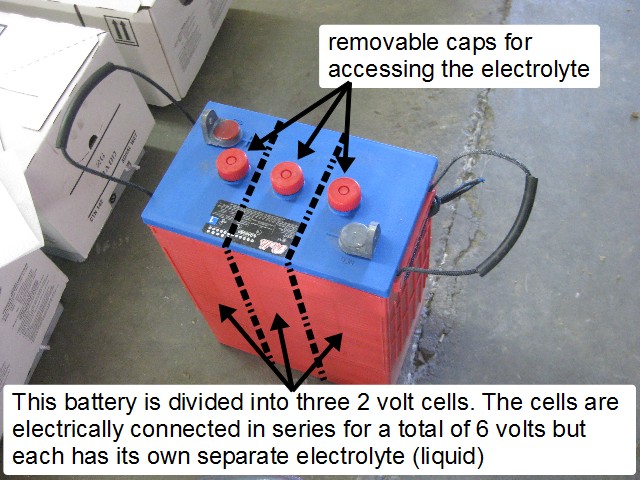
You can measure the specific gravity using a hydrometer if you have flooded lead acid batteries, ones with caps on top that you can remove to get at the liquid (electrolyte) inside. Then look up the specific gravity in the following table to find the Depth of Discharge (DOD) of the battery's cell that you took the electrolyte from. If you have sealed batteries then there are no removable caps and you can't do this.
| DOD | 2 volt battery | 12 volt battery | 24 volt battery | 48 volt battery | specific gravity |
|---|---|---|---|---|---|
| 0% | 2.10 | 12.70 | 25.40 | 50.80 | 1.265 |
| 10% | 2.09 | 12.58 | 25.16 | 50.32 | 1.249 |
| 20% | 2.08 | 12.46 | 24.92 | 49.84 | 1.233 |
| 30% | 2.06 | 12.36 | 24.72 | 49.44 | 1.218 |
| 40% | 2.05 | 12.28 | 24.56 | 49.12 | 1.204 |
| 50% | 2.03 | 12.20 | 24.40 | 48.80 | 1.190 |
| 60% | 2.02 | 12.12 | 24.24 | 48.48 | 1.176 |
| Discharged | 1.75 | 11.90 | 23.80 | 47.60 | 1.120 |
The electrolyte contains a mix of sulfuric acid and distilled water.
Warning: These batteries contain sulfuric acid. Always wear goggles and rubber or PVC gloves when working with them.
Readings will not be accurate if you've just added water. Wait a while before testing so that the new water has time to mix with the existing electrolyte.
Each battery is made up of one or more cells. In the photo below there are three cells. To access the electrolyte you simply remove the cap, typically by unscrewing it. Make sure you do not drop anything into a cell.

The best, easiest to use and an affordable type of hydrometer is one that is a sealed cylinder with a squeeze bulb at one end and a small, flexible tube at the other end (see diagram below.) Inside is a float, something that looks like what you'd see in a mercury thermometer. Make sure you get one that tells you the values for the specific gravity and doesn't have just colors on it. The following diagram shows how to use it.
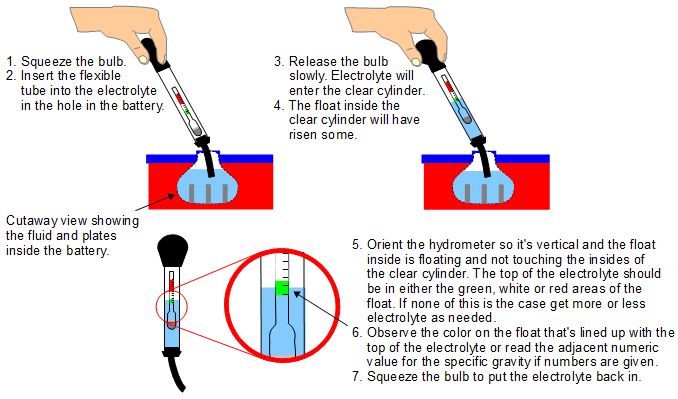
If the float in the hydrometer has numeric values on it for specific gravity, make a note of the value and the battery cell that you'd measured. If there are no values then the green indicates that the cell is charged, the white means it needs charging and the red means it's very discharged and needs charging but these are very rough indicators. Having the actual values is far preferable since you can compare the values for different cells and better monitor the health of each cell.
Temperature correction factors
The specific gravity will vary depending on the temperature inside the batteries. The manual for your batteries will tell you what correction to apply. For example, this is what the Surrette/Rolls manual says for temperatures in the range 0 to 130°F or -17.8 to 54.4°C. Use the equations below or for temperatures below 70°F (21°C) subtract 0.03 for every 10°F (5°C) that the temperature is below 70°F, and for temperatures above 70°F add 0.03 for every 10°F above 70°F.
- Correction factor (fahrenheit) = (0.331 x Battery_temperature_in_F - 23) / 100
- Correction factor (celsius) = (0.595 x Battery_temperature_in_C - 12.5) / 100
Many inverters or charge controllers have a battery temperature sensor that you attach to the battery bank somewhere to monitor temperature. They usually have an LCD display that you can query to find it out. Pointing an infrared thermometer gun at the side of one of the batteries in the middle of your battery bank will also give the temperature.
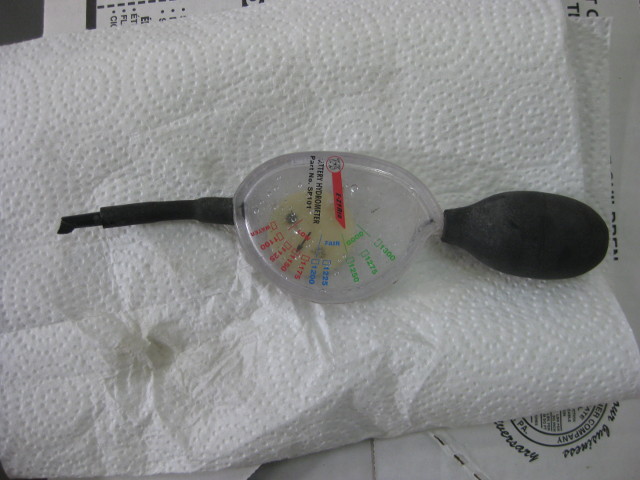
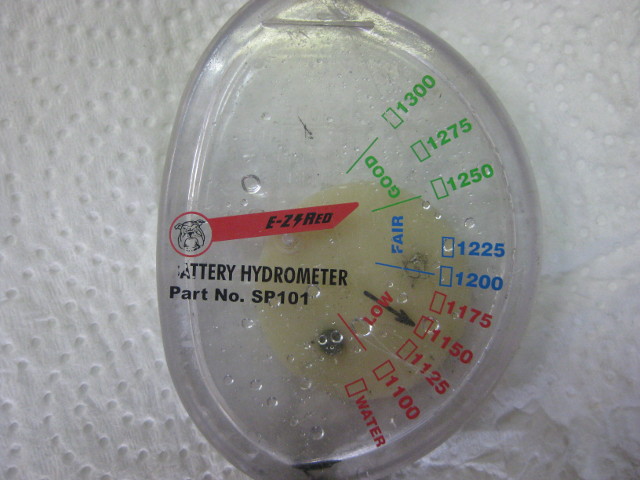
A dial-type hydrometer for measuring specific gravity
Below is another type of hydrometer with a dial instead of a float. It's a little less reliable because the dial may stick a bit when turning.