Peltier effect cooling efficiency test
A peltier module is a solid state device often used for doing cooling. Their found in some portable coolers for carrying food to the beach or in water dispensers like the one shown below.
While they're useful for those purposes, they're not very efficient. Only around 5% of the electrical energy used to power them gets used for cooling. I decided to do the simple efficiency test shown here. I wasn't testing the module directly, but instead testing how efficiently it could cool 250ml of water.
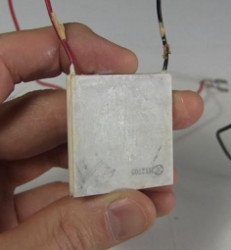

Shown below is the inside of the water dispenser from the back, and also the parts I took from it for this testing.

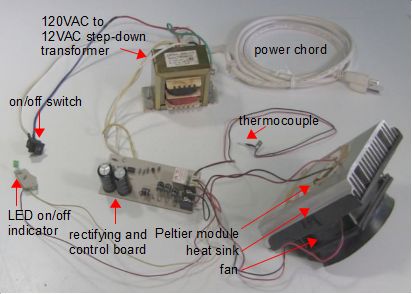
As shown below, the Peltier module was stuck to the back of the heat sink with thermal paste. The fan was attached to the other side of the heat sink to suck air through the heat sink's fins, carrying the heat away with it. The whole thing was sat up on jars so there'd be room below for the air to flow away.
You can also see that the thermocouple was inserted in a separate container of warm water. This was because the control board would shut off the Peltier module if the water at the thermocouple was the right temperature for a water dispenser. Since I wasn't caring about maintaining that temperature, I tricked it by sitting it in water whose temperature would never change. The result it that the control board wouldn't shut off the Peltier module for that reason.
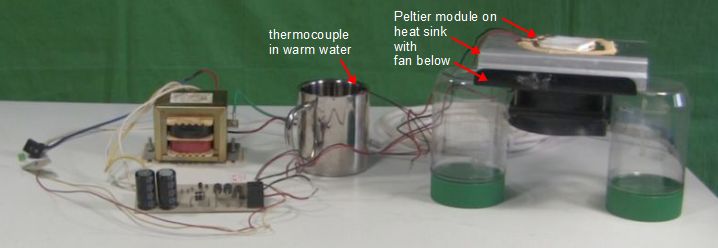
As shown below, I next sat a square piece of aluminum on the Peltier module to more effectively transfer heat from the can, which would be sitting on top of it, to all of the module's surface. I then sat a soda can on top of that. The top of the can was opened up and the bottom was flattened as much as possible. Next, insulation was added all around it. I wanted to take heat from the water in the can, not the surrounding air. I poured 250ml of water inside. A thermometer was then inserted inside, one that could measure cold temperatures. And lastly, the hole in the top of the can was covered with more insulation.






An oscilloscope was connected in parallel with the electrical output of the control board going to the Peltier module. An ammeter was connected in-line with the positive wires going from the control board to the Peltier module. The circuit was turned on using the on/off switch and the starting water temperature was recorded. The voltage and current had an initial few second long surge and then settled down. Their values were then recorded.
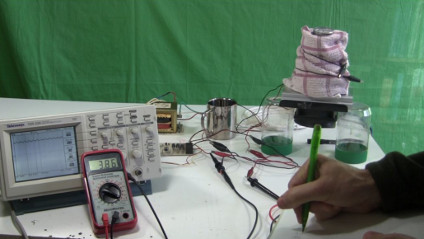
One hour later, the values were recorded again. The values were also looked at during the hour but not recorded. The voltage and current declined gradually during the hour, as did the temperature.
Peltier cooling efficiency calculations
250ml of water (0.55lbs)
Time |
Temperature Celsius |
Temperature Fahrenheit |
Voltage | Current |
|---|---|---|---|---|
| 3:55pm | 18C | 64.4F | 13.1V | 3.8A |
| 4:56pm | 14.5C | 58.1F | 12.8V | 3.66A |
input power = 13.1V * 3.8A = 49.8W = 49.8 joules/second
input energy = 49.8 joules/second * 3600 seconds = 179,280 joules
temperature change = 64.4F - 58.1F = 6.3F
BTUs used for cooling = 0.55lbs * 6.3F * 1BTU/lbF = 3.465 BTU
energy used for cooling = 3.465 BTU * 1055 joules/BTU = 3655.58 joules
efficiency = 3655.58 / 179,280 = 0.02039 = 2%
This is about what was expected. Peltier modules are only around 5% efficient. This means there was about 3% of additional losses.
Video - Peltier Module Cooling - The Peltier Effect
The following video shows taking the Peltier module from the above water dispenser and doing this Peltier cooling efficiency test. It has the additonal feature of showing a little water being turned to ice while sitting on the Peltier module for just a few minutes.
