Curie temperature experiment with ferromagnetism
This is a neat, simple science experiment involving the Curie temperature, a candle flame and a bit of nickel. The result is a little machine that has a piece of nickel swinging in and out of a candle flame with no obvious means of locomotion. A full explanation and how to make video is below.
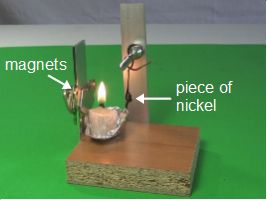

Here's an animated GIF (300kB) of the Curie temperature experiment in action.

How the Curie temperature experiment works - the science
A ferromagnetic material is one that can be magnetized by placing it in a magnetic field. Nickel is an example of such a material. The Curie temperature is a temperature at which a material magnetized in that way will lose it's magnetism. Nickel's Curie temperature is 358C (676F), meaning that when it reaches that temperature it loses its magnetism.
With that in mind, here's how the experiment works. As you can see in the photos above and in the drawings below, the piece of nickel is attached to the bottom of an arm which allows it to swing back and forth freely. Near it is a candle flame and beyond that is a magnet. At all times, the nickel is in the magnet's magnetic field.
1. Referring to the 1st diagram below, you can see the piece of nickel is made up of nickel atoms.
2. The nickel atoms act like tiny magnets, also called dipoles.
3. Those tiny magnets/atoms are grouped in domains. Domains are groups of magnets that are all oriented the same way. But notice in the diagram below that overall, the domains aren't all oriented in the same way. The result is that the piece of nickel is not magnetized i.e. it does not act like a magnet.
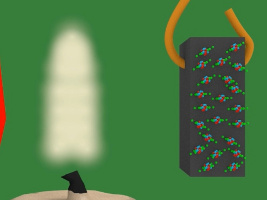
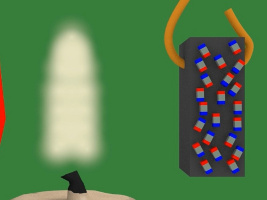
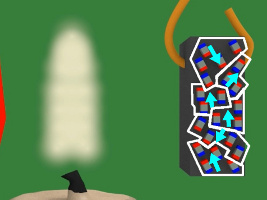
4. In the diagram below, the magnet is the blue and red object on the left. The white lines represent the magnetic field of that magnet. Notice that when the piece of nickel is first put in the magnetic field it is not magnetized, its tiny magnets/atoms are not all aligned.
5. But then due to the magnetic field, the tiny magnets line up with the magnetic field. The nickel becomes magnetized.
6. Since the nickel piece is now magnetized, it attracts to the magnet.
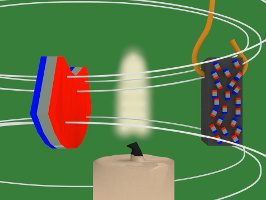
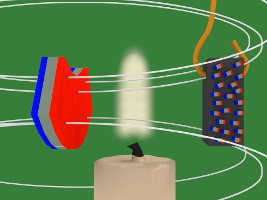
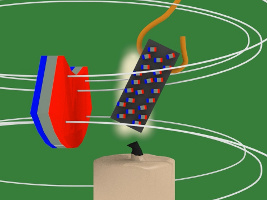
7. But there's a candle flame where the nickel has been attracted to. That flame heats up the nickel. Heat is defined as atoms or molecules moving randomly. The magnetic field resists this random motion. But eventually the temperature of the nickel reaches its Curie temperature/point (358C/676F) and the magnetic field loses the battle. The atoms start to move around randomly.
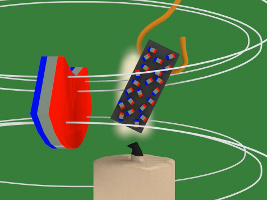
8. But that means the nickel's tiny magnets/atoms are no longer lined aligned meaning the nickel is no longer magnetized, and so it falls away from the hot flame.
9. Since it's no longer in the flame, the nickel cools again and becomes magnetized again, and it's attracted back to the magnet and into the flame and this keeps repeating.

Video - Curie Temperature/Point Experiment in Ferromagnetism - Science Short
The following video demonstrates the curie temperature experiment in action, explains how it works and shows how to make it step-by-step.

